ERC-Grant: THE ACTINIDE BOND - Actinide bond properties in gas, liquid and solid state
- Ansprechperson:
- Förderung:
European Research Council (ERC)
- Starttermin:
2021
- Endtermin:
2026
THE ACTINIDE BOND : Actinide bond properties in gas, liquid and solid state
Summary
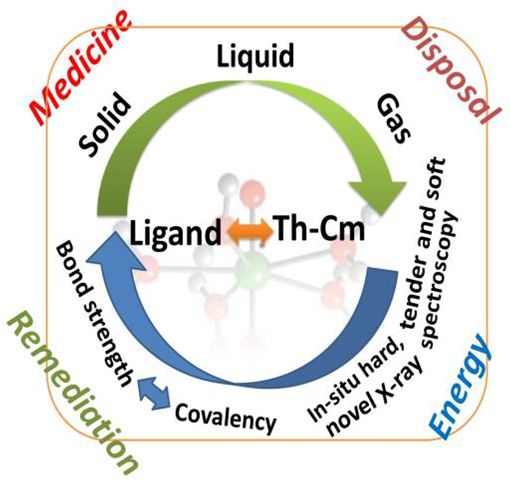
Understanding the electronic structure and chemical bonding properties of the early actinide (An) elements (Th-Cm) poses a great challenge and frontier in fundamental chemistry and physics. I aim to clarify the link between covalency and strength of the chemical bond of the early An elements from Th to Cm in gas, liquid and solid state materials - combining innovative high challenge experimental setups, advanced synchrotron based spectroscopy methods and state-of-the-art quantum chemical computations. In-situ structural studies of radioactive/radiotoxic gaseous and liquid An materials, combining soft and hard Xray scattering and absorption methods (RIXS/HR-XANES) to probe An metal centres and their ligands, have not yet been performed at any synchrotron light source in the world. The RIXS and HR-XANES methods probe the occupied and unoccupied parts of the valence band with extraordinary energy resolution and, when combined for the metal and the ligand, unique information on the chemical bond can be obtained. I will gain a deep understanding of the An bond formation mechanisms and will develop spectroscopy methodologies with high potential for a breakthrough in efforts, e.g., to select ligands and An materials with specific characteristics. Ligands and materials with tailored properties are needed for separation of An elements from chemically similar lanthanides or for developing advanced pharmaceutical compounds for targeted cancer treatment. A deep insight into the An electronic structures is also essential for developing innovative spent nuclear fuel matrices and to understand actinide environmental behaviour e.g. in contaminated sites. The new spectroscopy approaches are also expected to boost the advances of quantum chemical theoretical methods. Those are most challenged by the An atoms due to their large number of valence electrons and prevailing influence of relativistic effects in their electronic structure behaviour.